
Exhibit at AAHKS 2022
AAHKS 2022 Annual meeting is quickly approaching! The meeting will be held at the Gaylord Texan Resort & Convention Center, Grapevine, Texas, USA. And we are proud to be showcasing our innovative orthopedic implant and instrument portfolios being used to help restore joint mobility around the world at this very important meeting!
We are looking forward to seeing you in person at AAHKS in-November 3-5!
The U2 Knee System Meets ASC
It’s no secret that more surgeons than ever before want to perform total joint replacement (TJR)
procedures in outpatient settings instead of in hospitals. The forecasted number of outpatient TJR
procedures is based on the shift from inpatient dominance to outpatient growth. More than one
million TJR procedures are performed each year, and that number is expected to grow
exponentially, reaching four million by 2030 (see “National Billing Services,
nationalASCbilling.com, Info@nationalASCbilling.com”). A significant percentage of these cases
will migrate from inpatient to outpatient settings.
To ensure United Orthopedic Corporation continues to be one of the fastest-growing medical
device companies in the United States, we have developed a strategic ASC pricing model for our
innovative and award-winning U2 knee system – AIO (All-In-One) Femoral Block & MDT (Modular
Disposable Trials).
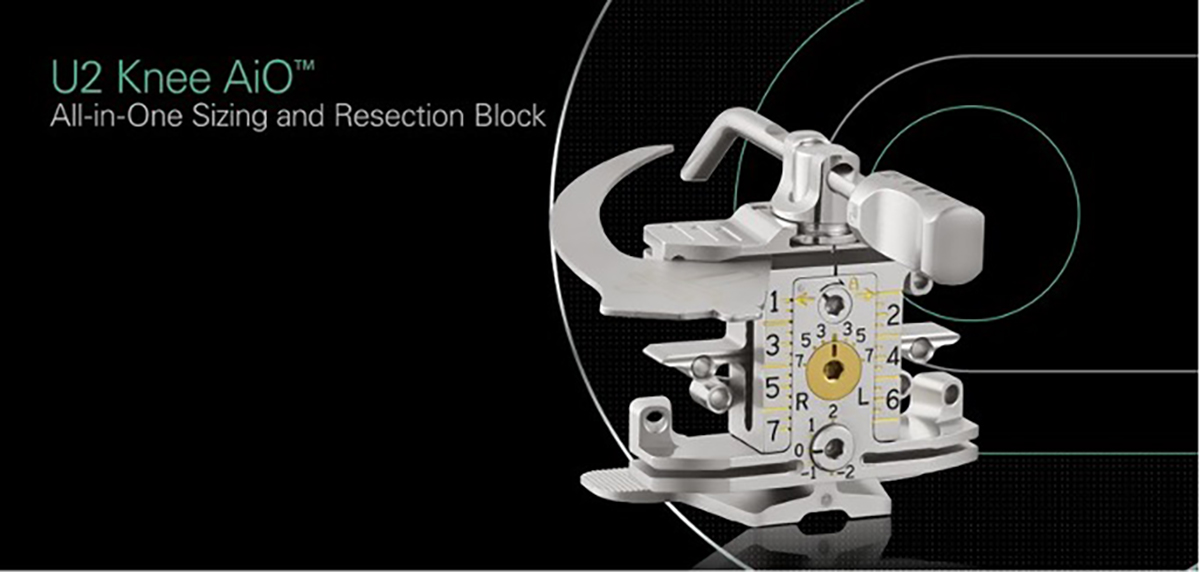
AiO (All-In-One) Femoral Block
The U2 Knee System’s AiO (All-in-One) femoral block enables surgeons to perform key femoral preparation
steps with a single instrument for a faster, more efficient, and precise surgical technique.
- Supports both anterior and posterior referencing
- Placed on the resected distal femur
- Permits internal/external rotation adjustments from 3 to 7° in 1° increment.
- Allows sizing of all 13 sizes of U2 femoral components
- Ultra-fine anterior-posterior (A/P) adjustment can be made from -2 mm to +2 mm for allowing for optimal component positioning
- Used for high-precision anterior and posterior femoral resections
- Sets the position for the anterior and posterior chamfer resection
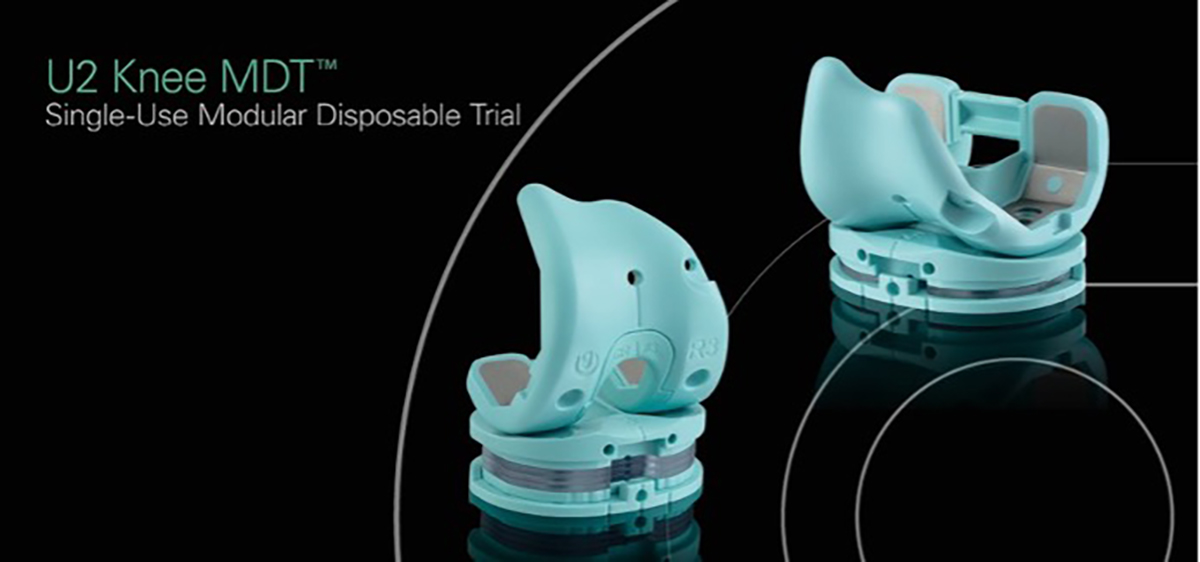
Modular Disposable Trials (MDT)
United’s Femoral Trial Set (CR/PS) is comprised of 7
standard sizes plus 6 intermediate sizes, for a total of
13 femoral component selections. Each sterile package
includes a CR notch trial & PS box trial to convert the
femoral trial component from a CR to a PS design. A
disposable PS Notch Cutting Guide is also included.

United’s Tibial Trial Sets (CR/PS or UC) are comprised
of 7 sizes of tibial baseplate trials and all thickness
selections of inserts. Each sterile package includes the
selected tibial baseplate trial, a standard 9 mm
articulating surface trial, three 2 mm spacers, and one
3 mm spacer. An additional PS post trial that allows
the CR insert trial to be converted to a PS insert trial is
also included.

United’s Patellar Trial Set Includes 7 size selections of
onset or inset patellar trials.

Opportunity
If you have ASC conversion opportunities and/or questions regarding the ASC pricing model, AIO
(All-In-One) Femoral Block, or MDT (Modular Disposable Trials), please contact your sales
management team.